|
Chapter 17 Application to Other Species | ||||||||||||||||||||||||||||||||||||||||
|
continued, Section 3 of 4 The Memory Criterion, by Entity Class: Restating the working definition:
Memory: "Memory" will stand for "episodic memory." This is the ability to "reach back" into the past. More specifically, it is the ability to retrieve egocentric episodes as a temporal chain of life events. Inanimates: Some inanimate bodies retain historical information
in their structure. A varve, or sedimented lakebed, may retain a record of
seasonal droughts as layers within the sediment. A lava rock may retain a
record of its age in the form of gas isotopes trapped within its matrix.
But inanimates themselves lack any means of retrieving these historical data
as useful memories. Among the inanimates found in nature, none satisfy the
memory criterion.
Eukaryotes/prokaryotes: Some mobile bacteria demonstrate a
short-term memory of their environments.[3] This
environmental memory helps the bacteria navigate towards food
sources. But this short-term memory is chemical, rather than neural, and is used
only for food-navigation. It can serve no other purpose.
Specifically, it cannot store long-term episodic memories.
Other
single-celled organisms operate under comparable memory constraints.[4] So no unicellular
organism satisfies the memory criterion.
Multi-celled
plants: Plants are sessile by definition. They lack the nervous
systems found in mobile animals. Lacking the nervous systems necessary for
perception, they cannot retain memories of life events. So among multi-celled
plants, none satisfy the memory criterion.
Computers: Computers store data in short-term memory structures,
such as RAM chips, and in long-term memory structures, such as disks and
tapes. But here our anthropomorphic tendencies can deceive us: most
"data" bear no correlation to the episodic memories germane to the
memory criterion. In the great majority of computers the stored data says
nothing about the computer itself. The information useful to humans is
useless to the computer because it is irrelevant to the computer's
"welfare," if we can use that word.
Only that data
which constitutes historical information about the computer's own structure and environment might
be considered relevant. This category probably covers "diagnostic
files" and "sensor inputs," for example. A
robotic computer could be expected to retrieve and interpret these memories in order to
repair a fault or execute some maneuver.
If the
diagnostic files and sensor inputs were stored as autoassociation patterns and
chained temporally, they could form a valid episodic memory trace;[5] and hence satisfy the memory
criterion. In fact, one such scheme has already been employed successfully in a mobile robot. Jun Tani has developed a neural net robot which
uses a visual episodic memory trace to navigate a dynamic maze.[6]
Still, most computer memory systems
clearly fail the test.
Invertebrates: Many invertebrates operate under a learning
constraint which puts them at a disadvantage relative to vertebrates:
| ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
Figure 17.1 highlights the difference. Invertebrate memory conditioning is shown at left, in (a). For the invertebrate, a teaching input ("unconditioned stimulus," or "UCS") must position its synapse on a single stimulus which is to be conditioned (CS). The teaching input conditions just that one stimulus. The vertebrate architecture,
shown at right in (b), is different. In
the vertebrate, a teaching input (UCS) can spread long distances along a
dendrite (in this case, vertically up and down the whole dendrite at
right). As a result, the vertebrate teaching input can condition many stimuli simultaneously.
The vertebrate
mechanism is the one appropriate for pattern association and autoassociation.[8] And autoassociation
enables storage and retrieval of episodic memories (memories of events).
Invertebrates lack that autoassociative mechanism, and hence lack episodic event
memory.
As a behavioral
example of this difference, we can contrast the ways in which vertebrates and
invertebrates learn mazes. Both can learn simple T-mazes. But an ant
taught a T-maze with one eye covered must relearn the maze when that cover is
removed and placed on the other eye.[9] No vertebrate would be hindered by such trickery. The
vertebrate's general-purpose associative memory store is isolated from the
individual sense organs. A vertebrate's memory of the maze is therefore a
memory of a series of events which can be recalled at will, independent of
sensory cues. Ants, like all insects, operate without
benefit of such an abstract and centralized memory store.[10]
Or, might there be exceptions to this rule?
The honeybee brain deserves special attention because the bee's foraging
technique requires an exceptionally robust memory system — perhaps the most
highly developed among insects. The honeybee brain contains a pair of
organs called "mushroom bodies," due to their mushroom-like
shape. They are the bee's primary organs for the acquisition of complex
memories.[11]
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
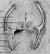
Figure 17.2 shows the position of the mushroom bodies (MB) inside the honeybee head. The scale at lower right indicates that the mushroom bodies have a combined diameter of about 1 mm. |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
Figure 17.3 shows details of the honeybee brain. Possible regions of long-term memory storage in the mushroom bodies are labeled as median calyx (mC) and lateral calyx (lC). The microstructure of these regions bears a resemblance to that of the vertebrate cerebellum.[14] We can see the similarity if we compare diagrammatic illustrations of the two structures. Figure 17.4 illustrates typical connections in the vertebrate cerebellum:
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
In Figure 17.4 parallel fibers (PF) can be seen synapsing on the dendrites of vertical climbing fibers (CF). A similar arrangement is visible in Figure 17.3, inside the a-lobe (aL) of the mushroom body at left. There the parallel fibers of Kenyon cells (K) appear to synapse on the dendrites of protocerebro-calycal tract (PCT) neurons. If function here
follows form, then the learning which occurs in mushroom bodies may be similar
to that known to occur in the cerebellum. This would be a kind of
"conditioned motor learning."[16]
This "motor learning" is less flexible than that of the general-purpose
autoassociator. The mechanism, while powerful, does not appear to be
capable of building the episodic memories required by personal identity.
Figure 17.5, below, illustrates diagrammatically the wide gap in function
separating motor memory (or "skeletal musculature memory") from
episodic memory (memory of events).[17]
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
This line of reasoning leads us to think that even advanced forms of insect invertebrate memory cannot satisfy the memory criterion of personal identity. |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
The octopus is the cephalopod most studied, and the one whose mental life is best understood. The octopus appears to use event memories in much the same way that vertebrates use memories recorded by the hippocampus. For example, among vertebrates the hippocampus is vital to mapmaking. Octopus vulgaris has also been observed to engage in mapmaking. Field observations suggest that this octopus follows a detailed topographical map when navigating the coral reef near its den.[22] Figure 17.7 traces some typical octopus foraging trips:
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
During each trip the octopus departs from its den, navigates to a foraging area, and then returns to its den. Note in Figure 17.7 the directness of the final leg of each trip: the foraging octopus, when startled, darts directly home.
An animal follows a direct path, and ignores landmarks, when it knows the
direction to its destination. The octopus' vertebrate competitors use a mental map to obtain that knowledge.
The same is likely true of the octopus. In functional
terms, Octopus vulgaris' vertical lobe seems to be
autoassociating optical views of the reef into a unified mental map.
Octopus vulgaris has also demonstrated the ability to retain
arbitrary T-maze memories over a long period of time in the laboratory.[24] This ability is
comparable to that exhibited by the lower vertebrates.
By the example
of Octopus vulgaris we can say that a few
cephalopod invertebrates do appear to satisfy the memory criterion.
Vertebrates: The function of the human hippocampus was sketched
in Chapter 6. The hippocampus stores and retrieves episodic
memories.
The hippocampus
is not an organ new to the human brain. It is actually an ancient organ
found in all vertebrates, from early fishes to man.[25]
Much theoretical work on the human
hippocampus (such as that presented in Chapter 6)
has actually been based on studies of the hippocampus in lower
vertebrates, especially rats. The hippocampus is a ubiquitous
vertebrate organ of memory: one which records and retrieves episodic memories
for all vertebrate species. It follows that all vertebrates most likely do satisfy the
memory criterion.
Some labeled
cross-sections and diagrams can illustrate the commonality of hippocampal
structures among vertebrates:
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
In Figure 17.8 the hippocampus is marked in black. "A" is the condition in monotremes and marsupials; "B" is a hypothetical intermediate stage; "C" is the condition in the hedgehog; "D", the bat; "E", the rodent; "F", primates and other advanced mammals. |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
In support of the theoretical unity of vertebrate memory, we can note that several fish species have demonstrated an ability to learn simple mazes. The ability is degraded by ablation of higher brain centers.[28] Similar abilities and ablation impairments have been recorded throughout the vertebrate sub-phylum.[29],[30] |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
Mammals: As with vertebrates generally. All mammals satisfy the memory criterion. |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
Great apes: As with mammals generally. All great apes satisfy the memory criterion. |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
Humans: All
humans satisfy the memory criterion.
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
The column for memory has now been filled
in. We can proceed to the final criterion of personal identity —
subjectivity.
next Section 4 of 4 | ||||||||||||||||||||||||||||||||||||||||
|
Chapter 17, Section 3 Endnotes |
||||||||||||||||||||||||||||||||||||||||
|
[3] D. E. Koshland, Jr., "A Response Regulator Model in a Simple
Sensory System,"
Science 196:4294 (1977):
1055-63.
[4] R. Menzel and G. Bicker, "Plasticity in Neuronal Circuits and
Assemblies of Invertebrates, "The Neural and
Molecular Basis of Learning, eds. J.-P. Changeux and M. Konishi (Chichester:
John Wiley & Sons, 1987) 449. Quoting:
"There is no firm evidence for associative learning in unicellular organisms such as protozoans or 'brainless' multicellular organisms such as the coelenterates, whereas nonassociative plasticity is found in both phyla." [5] For some recent neural net memory models see Edmund T. Rolls,
"A Theory of Hippocampal Function in Memory," Hippocampus 6:6 (1996): 601-20; Levy
579-90; Moll and Miikkulainen 1017-36. For theory and experiment concerning temporally-ordered memory
recall, see Levy 579-90. See also August and Levy 231-36; Qin et al.
1525-33.
[6] Jun Tani, "An Interpretation of the 'Self' from the Dynamical Systems Perspective:
A Constructivist Approach," Models of the Self, eds. Shaun Gallagher and Jonathan Shear (Thorverton, UK: Imprint Academic, 1999) 149-76. For details of the robot's
hippocampal analogue, see page 162.
[7] Rolls and Treves 34.
[8] A "pattern associator" learns to map an input pattern to
an output pattern. An "autoassociator" is a special type of
pattern associator, which learns to map an input pattern as its own
output. See Rolls and Treves 33-36 for an analysis of possible differences between
vertebrate and invertebrate association mechanisms.
[9] Theodore Holmes Bullock and G. Adrian Horridge, eds., Structure and Function in the Nervous Systems of
Invertebrates, 2 vols. (San Francisco: W. H. Freeman and Company, 1965) 1:
337. This laboratory observation has been verified in the field. See Rudiger Wehner
and Martin Muller, "Does interocular transfer occur in visual navigation
by ants?" Nature 315 (1985): 228-29. For details of robust interocular transfer
in mammals, see Giovanni Berlucchi, "Interaction of Visual Cortical Areas and
Superior Colliculus in Visual Interhemispheric Transfer in the Cat," Changing Concepts of the Nervous System,
eds. Adrian R. Morrison and Peter L. Strick (New York: Academic Press, 1982)
321-36. For a comparison of maze learning in the ant and the rat, see Richard A. Maier,
Barbara M. Maier, Comparative
Animal Behavior (Belmont: Brooks/Cole Publishing Company, 1970) 293-98.
A similar limitation of insect memory has been observed in honeybee olfaction.
Bees trained to associate an odor on one antenna exhibit no
associated memory of that odor when it is presented to the other antenna.
This effect has been described in R. Menzel, J. Erber, and T. Masuhr,
"Learning and Memory in the Honeybee,"
Experimental Analysis of Insect Behaviour, ed. L. Barton-Brown (New York:
Springer Verlag, 1974) 195-217. R. Menzel has analyzed the effect in situ, in R. Menzel, "Memory Traces in
Honeybees," Neurobiology and Behavior of Honeybees,
eds. Randolf Menzel and Alison Mercer (Berlin: Springer-Verlag, 1985)
310-25.
[10] For surveys of the upper limits of insect learning, see B.
Heinrich, "Learning in Invertebrates," The
Biology of Learning, eds. P. Marler and H. S. Terrace (Berlin:
Springer-Verlag, 1984) 135-47; and from that same text; J. L. Gould,
"Natural History of Honey Bee Learning" 149-80. See also Menzel
and Bicker 433-72.
[11] Jochen Erber, and Uwe Homberg, "Neural Signal Processing in
the Median Protocerebrum of the Bee," Neurobiology
and Behavior of Honeybees 253-64.
[12] Jurgen J. Milde, "The Ocellar System of the
Honeybee," Neurobiology and Behavior of
Honeybees 192. Quoting the original caption:
"Frontal view of a bee's head with a window cut into the head capsule. Outlines of prominent brain structures are indicated. A single L-neuron from the median ocellus can be seen behind the central complex (CC). AL = antennal lobe; Ant = antenna; CE = compound eye; Lob = lobula; MB = mushroom body; Med = medulla; MOC = median ocellus; O = oesophagus." [13] Menzel and Bicker 458. Quoting the original caption:
"The brain of the honeybee. MC and lC: median and lateral calyx of the m.b.; aL: alpha lobe of the m.b.; K: Kenyon cells, the intrinsic neurons of the m.b.; mAGT and lAGT: median and lateral antenno-glomerularis tract; PCT: protocerebro-calycal tract; Oc: ocelli; AL: antennal lobe; Soe: subesophageal ganglion; AN: antennal nerve; MN: motorneuron to muscles moving the proboscis (tongue); OL: optic lobes." [14] Friedrich-Wilhelm Schurmann and Karoly Elekes, "Synaptic
Connectivity in the Mushroom Bodies of the Honeybee Brain: Electron Microscopy
and Immunocytochemistry of Neuroactive Compounds," Neurobiology and Behavior of Honeybees 225-34. See especially
the direct comparison with the cerebellum on page 227. See also Menzel and
Bicker 457.
[15] Rolls and Treves 192.
[16] For an example of cerebellar conditioned motor memory acquisition
in humans, see Fuster 163-64.
[17] Illustrated memory types are those known in mammals.
[18] Milner, Squire, and Kandel 451.
[19] Roger T. Hanlon and John B. Messenger,
Cephalopod Behaviour (Cambridge: Cambridge University Press, 1996) 184.
[20] For a survey of associative learning in the octopus, see Hanlon
and Messenger 138-48. See Hanlon and Messenger 27-29 for details of
long-term memory storage in the optic lobes. For an earlier and
complementary interpretation of vertical lobe functions, see M. J. Wells, Brain and Behaviour in Cephalopods (Stanford: Stanford
University Press, 1962) 111-41.
[21] Hanlon and Messenger 28.
[22] Hanlon and Messenger 144-45. Recent experimental evidence
indicates that honeybees also create mental maps of their foraging areas.
The map images stored by honeybees are of a low resolution, in accord with the
limited memory capacity of their brains' mushroom bodies (1mm combined
diameter). However, the honeybee's ability to make even crude visual maps
is truly remarkable, considering that bees lack the auto-association brain center
(hippocampus) so important to vertebrate mapmaking. For details of
honeybee mapmaking, see Gould 298-309. For details of vertebrate mapmaking, see
N. Burgess, K. J. Jefferey, and J. O'Keefe, eds., "What are the parietal
and hippocampal contributions to spatial cognition?" Philosophical Transactions of the Royal Society of London: Series B
352:1360 (1997): 1395-1543.
[23] Hanlon and Messenger 145.
[24] Hanlon and Messenger 140-41. See also, octopus learning by observation, in the following section.
[25] Harvey B. Sarnat and Martin G. Netsky,
Evolution of the Nervous System, 2nd edition
(Oxford: Oxford University Press, 1981) 338-41.
[26] Sarnat and Netsky 342. Quoting original caption:
"Diagram of medial aspect of cerebral hemisphere to show the evolution of the corpus callosum and septum pellucidum. (A) condition in monotremes and marsupials: hippocampus (black) and subiculum (stippled) lie dorsal to lamina terminalis; (B) hypothetic intermediate stage: hippocampal infolding brings subiculum closer to lamina terminalis; broken line in subiculum indicates position of incipient fibers of corpus callosum; (C) condition in hedgehog and bat: corpus callosum develops by penetrating subiculum; most of hippocampus beneath corpus callosum is obliterated; (D) except for small precommissural remnant, hippocampus lies entirely behind corpus callosum; (E) condition in rodents: splenium of corpus callosum expands as more fibers are needed; (F) condition of primates and other advanced mammals: rostral portion of corpus callosum expands and forms an arc, drawing the frontal part of lamina terminalis into the concavity to become part of the septum pellucidum; neural component is derived from paraterminal body. Subiculum above corpus callosum is induseum griseum in man. See text for further details. Arrows indicate direction of expansion; broken lines in the septum pellucidum are successive positions of the genu of the corpus callosum. Anterior commissure (CA); corpus callosum (CC); inferior fornix (FI); superior fornix (FS); lamina terminalis (LT); paraterminal body (PtB); rostrum (Ros); splenium (Spl); septum pellucidum (SL); subiculum (Sub). (Abbie, 1939)" [27] Sarnat and Netsky 410.
[28] George Eric Savage, "The Fish Telencephalon and Its Relation
to Learning," Comparative Neurology of the
Telencephalon, ed. Sven O. E. Ebbesson (New York: Plenum Press, 1980)
149-50.
[29] The vertebrate sub-phylum is a branch of the phylum
Chordata, which groups notochordate animals together with true vertebrates.
[30] For details of mapmaking and map utilization in the rat, see Gene
V. Wallenstein, Howard Eichenbaum, and Michael E. Hasselmo, "The
Hippocampus as an Associator of Discontiguous Events," Neurosciences 21:8 (1998): 317-23.
An experimental validation of associative mapmaking in the rat can be found in
David McFarland, Animal Behavior: Psychobiology,
Ethology and Evolution (Menlo Park: The Benjamin/Cummings Publishing
Company, Inc., 1985) 354-56.
For details of mapmaking in the
mountain chickadee, see Nicola Clayton, "Episodic Memory in Mountain
Chickadees," Neuropharmacology
37 (1998): 441-52. See also Nestor A.
Schmajuk, Animal Learning and Cognition (Cambridge:
Cambridge University Press, 1997) 219-40.
[31] Sarnat and Netsky 410.
[32] Sarnat and Netsky 410.
[33] Ronald Pearson and Lindsay Pearson, The
Vertebrate Brain (London: Academic Press, 1976) 611.
[34] Pearson and Pearson 606.
[35] Sarnat and Netsky 409.
[36] Sarnat and Netsky 410.
[38] Robert L. Isaacson and Karl H. Pribram, eds., The Hippocampus, Vol. 1: Structure and Development, 4 vols. (New York:
Plenum Press, 1975) 1: 42.
[39] Sarnat and Netsky 408.
|
Copyright © 1999
Wayne Stewart
Last update 4/19/11