|
Chapter 6 Episodic Memory | ||
|
The Church-Turing Thesis, abbreviated here
as CT, applies recursive functions to common
computing problems. Most of these problems are solved by "serial
computers," which execute instructions in a linear sequence, one by
one. CT also applies to computers which
execute instructions in parallel, performing many
computations concurrently. John Shepherdson[1] has proved the applicability of CT
to parallel computers.[2]
Some parallel computer designs are inspired by the microscopic architecture of
neural structures in the brain. Parallel computers known as "neural
nets" are built so as to mimic these structures. Since we're
chasing CT into the human body, it will
be expedient to restrict pursuit of CT applications to neural nets alone.
Recently neural
nets have been refined so as to simulate the electrical "spiking"
behavior common to real neurons. The spiking model places
additional biological constraints on neural nets,[6] making
modern versions more realistic than the early 40s
model of McCulloch and Pitts. Like those early neural nets, spiking
neural nets can also simulate all Turing machines effectively. In addition,
Wolfgang Maas[7] has
demonstrated that spiking neural nets can simulate other classes of
machines, including finite automatons[8] and random access memory devices.[9]
And very recently (1997) Maas[10] has
shown that spiking neural nets can achieve computational
performance far superior to that of McCulloch-Pitts neural nets.[11]
These results
must be encouraging for theoreticians, but they remain very much
theoretical. The models are inspired by neural anatomy; however, very few simulate anatomic structures in detail.
More relevant
results would be those which map to particular
recursive structures in the human brain. If these structures could be
shown to exhibit powers transcending mere self-motion (preferably rising towards
the psychological realm) the results would be of value to the current
critique. We will see below that a brain structure essential to the
function of memory does exhibit such powers.
The hippocampus
is not an easy structure to visualize. For this reason several anatomical
images will be presented among the paragraphs to follow. The images
are ordered so as to guide the reader's eye down to the hippocampus, and to
expose the recursive structure within.
|
||
|
||
|
This cutaway view displays some of the brain's deep limbic system structures, as seen through the temporal lobe from a viewpoint above the left temple. The two lobes of the hippocampal formation lie below the centrally-located thalamus. Distant brain areas connect to the hippocampus through two fiber bundles visible in the illustration: the cingulate gyrus and the fornix. The cingulate gyrus contains most of the hippocampus' afferent (input) fibers. The fornix contains most of the efferent (output) fibers. |
||
|
||
|
Figure 6.1 is inset within Figure 6.2, at upper left. Figure 6.2 isolates the hippocampal formation (shaded in inset). This cutaway reveals the folded tissues of the hippocampus. The dentate gyrus is the interior fold. The cornu ammonis is the exterior fold. The cornu ammonis is not labeled in Figure 6.2, but is marked in another cutaway view, Figure 6.3, below. |
||
|
||
|
This exterior fold, the cornu ammonis, is the main site of memory processing in the hippocampus. An overview of its supposed function will follow these figures. |
||
|
||
|
In Figure 6.4 the hippocampus is rotated 180 degrees relative to Figure 6.3. The bar at lower right is 6.5 mm, for scale. |
||
|
||
|
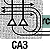
In Figure 6.5 the cornu ammonis is again labeled "CA." Two regions are marked: "CA1" and "CA3." Also, the dentate gyrus, at bottom, is once again labeled "DG." Major nerve pathways through these structures are illustrated by individual neurons. Arrows indicate the general flow of information. |
||
|
||
|
|
||
|
||
|
Figure 6.7 magnifies Figure 6.5 with a more detailed schematic of connections and information flow in the hippocampus. Note the looping structure marked as "Recurrent collateral" in the CA3 region at left. Recurrent collaterals occur where nerve output fibers (axons) circle back upon the nerve input fibers (dendrites) of neighboring cell bodies. Collaterals are the synaptic connections between axons and dendrites. The recurrent collateral in Figure 6.7 is of special interest: it is a known example of a recursive structure in the brain. We can trace the flow of information through the hippocampus, marking the recursion where it occurs: In
Figure 6.7 information flows through the perforant path (1) to the dentate gyrus
and the CA3 region. The dentate gyrus also projects information through
the mossy fibers (2) to the CA3 region. Recurrent collaterals cycle inputs
within the CA3 region. (This is the
recursion.) Thereafter the CA3 region projects through the Schaeffer
collateral (3) to the CA1 region. The CA1 region projects to the
subiculum (4) and on to other cortical areas.
The recursion in region CA3 figures more prominently
in Edmund Rolls' network schematic, Figure 6.8:
|
||
|
||
|
In Figure 6.8 the block diagram at left shows the overall information flow between regions. The symbolic diagram at right highlights recurrent collaterals ("rc") of the CA3 region, which stand out by virtue of their distinctive looping structure. The diagrams in Figure 6.8
clarify Figure 6.7. They showcase the hippocampus' recursive information
flow. Afferent (input) connections are shown as solid lines.
Efferent (output) connections are shown as dashed lines. At right, the more detailed
symbolic diagram shows dendrites as thick lines, and axons as thin.
"rc" indicates that recurrent axons loop their collaterals back onto
dendrites within the CA3 region — the site of recursion.
The role of the
hippocampus in storing and recalling events has been a subject of
investigation since the 1950s.[22] More recently the CA3 region has been singled out for
especially intense study. The CA3 region is the portion of the hippocampus
which contains a high number of recurrent collaterals, as noted in Figures 6.7
and 6.8. As a group, these "coiled" neurons produce outputs
which contribute to their own inputs. And under the right conditions
recurrent collaterals can propagate nerve impulses in self-sustaining
loops. This does seem to occur in the CA3 region. It's
a biological form of recursion, with computational properties
essential to the formation of episodic memories.
The computational properties
of greatest interest are those of an "autoassociator." This
device is just a neural net which has been completely permeated by its recurrent
collaterals.
|
||
|
||
|
Figure 6.9 diagrams an abstract autoassociator. The sequence of changes which are thought to occur during episodic memory formation in the CA3 region can be read from the diagram in this way:[24]
External input sensations (ei) excite dendrites (thick lines) to produce
simultaneous electrical activation (hi) on a group of neurons. In turn, those activated
neurons' cell bodies (triangles) fire outputs (ri). The recurrent collaterals (r'j) of these outputs
strengthen synaptic connections (wij) on just the excited dendrites in the group. This cycle
continues for the duration of the external input. After a few cycles the
memory pattern is stored, as a single remembered event, in the strengthened
connections.
Edmund Rolls and Alessandro Treves have modeled
the neural net thought to exist within the hippocampus.[25] Here are some of
their experimental results:
Rolls characterizes the results:
Other recent experiments support these
findings.[33],[34]
So we can infer with some confidence that the body relies heavily upon
recursion when storing and recalling memories of events.
This result,
and the results discussed previously, must now be laid out alongside
Proclus' propositions so that we may conclude this rambling critique.
next Chapter 7: Mortality | ||
|
Chapter 6 Endnotes |
||
|
[1] John C. Shepherdson, "Mechanisms for Computing Over Arbitrary
Structures,"
The Universal Turing Machine: A
Half-Century Survey 581-601. [2] Shepherdson 597. Quoting:
"For every mechanism satisfying [Gandy's four 'Principles of Mechanisms'] there is a parallel Turing machine which is equivalent to it on all interpretations on which it is determinate." [3] McCulloch and Pitt's original 1943 article, "A Logical
Calculus of the Ideas Immanent in Nervous Activity," is reprinted in Warren
S. McCulloch,
Embodiments of Mind (Cambridge,
Massachusetts: The MIT Press, 1965) 19-39.
[5] Hava T. Siegelmann and Eduardo D. Sontag, "On the
Computational Power of Neural Nets," Journal of
Computer and System Sciences, 50:1 (1995): 132-50.
[6] The "spiking" behavior is codified by complex threshold
and response functions. See also Chapter 17, note 42.
[7] Wolfgang Maas, "Lower Bounds for the Computational Power of
Networks of Spiking Neurons," Neural
Computation 8:1 (1996): 1-40.
[8] Finite automaton (finite state machine):
"an abstract machine consisting of a set of states
(including the initial state), a set of input events, a set of output events,
and a state transition function. The function takes the current state and an
input event and returns the new set of output events and the next state. Some
states may be designated as 'terminal states'. The state machine can also be
viewed as a function which maps an ordered sequence of input events into a
corresponding sequence of (sets of) output events." Source: Free On-line Dictionary of Computing.
[9] Random access memory:
"the most common computer memory which can be used by programs to perform
necessary tasks while the computer is on; an integrated circuit memory chip
allows information to be stored or accessed in any order and all storage
locations are equally accessible." Source: WordNet 1.6.
[10] Wolfgang Maas, "Networks of Spiking Neurons: The Third
Generation of Neural Network Models," Neural
Networks 10:9 (1997): 1659-71.
[11] The particular example chosen used a single spiking neuron to
perform a computation which would require hundreds of McCulloch-Pitts
neurons.
[12] Maas, "Lower Bounds for the Computational Power of Networks
of Spiking Neurons," 30 -31.
[13] Walter J. Hendelman,
Student's Atlas of
Neuroanatomy (Philadelphia: W. B. Saunders, 1994) 181.
[14] Hendelman 189.
[15] Peter McLeod, Kim Plunkett, and Edmund T. Rolls, Introduction to Connectionist Modelling of Cognitive Processes
(Oxford: Oxford University Press, 1998) 279.
[16] Henri M. Duvernoy, The Human
Hippocampus, 2nd ed. (New York: Springer,
1997)15.
[17] Juaquin M
. Fuster, Memory in the Cerebral Cortex: An Empirical Approach to Neural Networks
in the Human and Nonhuman Primate (Cambridge, Massachusetts: The MIT Press,
1995) 26.
[18] Duvernoy 19.
[19] McLeod, Plunkett, and Rolls 282. Quoting the original
caption:
"A schematic representation of connections within the hippocampus. Input comes through the perforant path (1) which synapses with the dendrites of the dentate granule cells and also with the apical dendrites of the CA3 pyramidal cells. The dentate granule cells project via the mossy fibres (2) to the CA3 pyramidal cells. The recurrent collateral system of the CA3 cells is indicated by the single axon labelled Recurrent collateral. The CA3 pyramidal cells project via the Schaeffer collateral (3) to the CA1 pyramidal cells, which in turn have connections (4) via the subiculum back to other cortical areas."
[20] McLeod, Plunkett, and Rolls 284. Quoting the original
caption:
"Forward connections (solid lines) from areas of cerebral association neocortex via the parahippocampal gyrus and perihinal cortex, and the entorhinal cortex, to the hippocampus. Backprojections (dashed lines) via the parahippocampal gyrus to the neocortex. There is great convergence in the forward connections down to the single network in the CA3 pyramidal cells and great divergence again in the backprojections. Left: block diagram. Right: more detailed representation of some of the principal excitatory neurons in the pathways. [triangle] represents pyramidal cell bodies; [circle] represents dentate granule cells. The thick lines above the cell bodies represent the dendrites; the thinner lines with arrow heads the axons. Abbreviations: DG: dentate granule cells. mf: mossy fibres. PHG: parahippocampal gyrus. pp: perforant path. rc: recurrent collateral of the CA3 hippocampal pyramidal cells."
[21] For an introductory overview of the principles of neural memory
formation, and the role of the hippocampus in the acquisition of episodic
memories, see Fuster 23-46. For photographs of long-term memory structures, see a dissection of memory structures in the sheep brain.
[22] For a very readable history of twentieth-century memory research,
see Brenda Milner, Larry R. Squire, and Eric R. Kandel, "Cognitive
Neuroscience and the Study of Memory,"
Neuron
20:3 (1998): 445-68.
[23] Edmund T. Rolls and Alessandro Treves,
Neural Networks and Brain Function (Oxford: Oxford University Press,
1998) 43.
[24] A good introduction to autoassociators can be found in McLeod,
Plunkett, and Rolls 72-95.
[25] Rolls and Treves 95-135. A summary of that analysis is
presented as part of an introductory course on neural net theory in McLeod,
Plunkett and Rolls 279-92.
[26] McLeod, Plunkett and Rolls 289.
[27] McLeod, Plunkett and Rolls 289.
[28] McLeod, Plunkett and Rolls 290.
[29] Rolls and Treves 113.
[30] Rolls and Treves 113.
[31] Rolls and Treves 122-29. For biochemical details, see Ivan
Izquierdo and Jorge H. Medina, "Memory Formation: The Sequence of
Biochemical Events in the Hippocampus and Its Connection to Activity in Other
Brain Structures," Neurobiology of Learning and
Memory, 68:3 (1997): 285-316. For a very recent (2000) proposed linkage of hippocampal and cortical memory systems,
see Edmund T. Rolls, "Hippocampo-Cortical and Cortico-Cortical Backprojections," Hippocampus, 10:4 (2000): 380-88.
[32] McLeod, Plunkett and Rolls 289-90.
[33] Some supplementary theories on overall hippocampal function can
be found in L. R. Squire, "Memory and the Hippocampus: A Synthesis From
Findings With Rats, Monkeys, and Humans,"
Psychological Review 99:2 (1992): 195-231; James L. McClelland and Nigel H.
Goddard, "Considerations Arising From a Complementary Learning Systems
Perspective on Hippocampus and Neocortex," Hippocampus 6:6 (1996): 654-65; Mark Moll and Risto Miikkulainen,
"Convergence-Zone Episodic Memory: Analysis and Simulations,"
Neural Networks 10:6 (1997): 1017-36; Richard Granger,
et al., "Distinct Memory Circuits Composing the Hippocampal Region," Hippocampus 6:6 (1996): 567-78.
[34] Some theories on the hippocampus' role in storage and retrieval
(including dreamtime retrieval) of temporal
sequences of episodic memories can be found in William B. Levy,
"A Sequence Predicting CA3 Is a Flexible Associator That Learns and Uses
Context to Solve Hippocampal-Like Tasks."
Hippocampus 6:6 (1996): 579; D. A. August and William B. Levy,
"Spontaneous Replay of Temporally Compressed Sequences by a Hippocampal
Network Model,"
Computational Neuroscience: Trends
in Research, 1997, ed. James M. Bower (New York: Plenum Press, 1997) 231-36;
Yu-Lin Qin et al.. "Memory in Hippocampal-Neocortical Ensembles," Philosophical Transactions of the Royal Society of London:
Series B 352:1360 (1997): 1525-33; J. L. Kavanau, "Memory, Sleep and
the Evolution of Mechanisms of Synaptic Efficacy Maintenance,"
Neuroscience 79:1 (1997): 7-44.
|
Copyright © 1999
Wayne Stewart
Last update 4/19/11